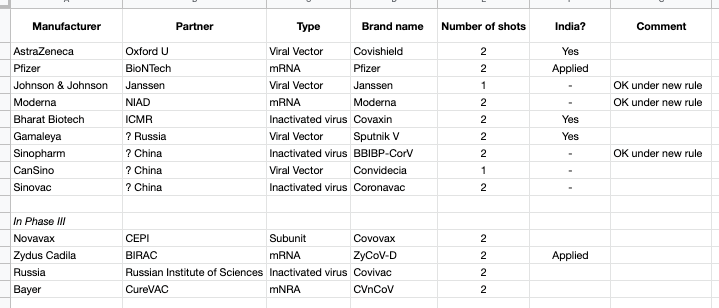
Because there were a lot of Covid-19 vaccine names mentioned in the Indian and other press, I put together a list of vaccines that appear to be in the market or in Phase 3 trials as of 10 May 2021.
The WHO has a basic primer on the different types of Covid vaccines.
I recently read a detailed two-part article exploring the constraints holding back vaccine manufacturing and distribution. Written by a professor of Law at Yale. Part One, Part Two.
The material focuses on patent law, and is probably the writer’s expertise.
But I am also interested in understanding manufacturing constraints: how long it takes for existing manufacturers to ramp up vaccine facilities, build new ones and repurpose other vaccine/drug facilities for a Covid vaccine. From a Financial Times article:
While some EU leaders do not want to appear intransigent on the IP waiver issue, they mostly agree that it is a distraction from the more pressing issue of expanding near-term production capacity through licensing deals and sharing technology… But that puts the onus on the EU to come up with industrial solutions for increasing production and for sharing its own supplies, due to expand vastly in the second half of the year, with developing countries.
– EU is the vaccine good guy that manages to look like the villain
After all, India’s SII already has a license and has transferred tech for the AstraZeneca vaccine. Another company, along with a couple of government agencies, has developed an effective indigenous vaccine branded Covaxin. A third pharmaceutical firm has licensed the Russian-origin vaccine, Sputnik V, but even after having been cleared for administration in India on 13 April, as of 13 May, a fresh round of ‘local testing’ means there is no clarity on when distribution will begin, despite 150,000 doses having arrived from overseas on 1 May.
So. Licensing has not alleviated the vaccination crisis in India.
While there are legitimate and necessary debates about granting other vaccine makers the rights to the locally-created vaccine, the high lead time to expand production capacity means a terrible slowdown in India’s vaccination rollout:
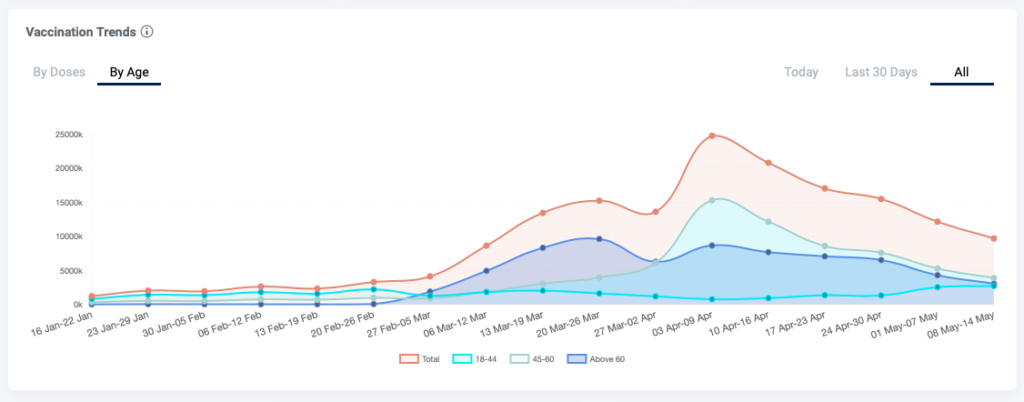
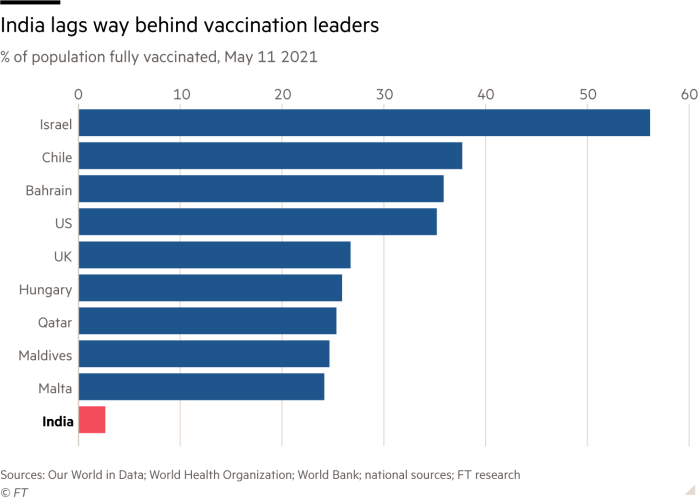
and has meant that despite being home to the world’s largest manufacturer, despite having a vaccine not limited by global IP rules, despite having a decentralised distribution mechanism, despite having no anti-vaxx movement, vaccination coverage in India is dismal.
(Part 2: pitting the centre, states and cities against each other)